1. WHAT IS MINOXIDIL SANDOZ 5%, solution for cutaneous application AND WHAT IS IT USED FOR?
Pharmacotherapeutic class
OTHER DERMATOLOGICAL PREPARATIONS.
Therapeutic indications
This medicine is indicated in cases of moderate hair loss (androgenetic alopecia) in males.
2. WHAT YOU SHOULD KNOW BEFORE USING MINOXIDIL SANDOZ 5%, solution for cutaneous application
List of information needed before taking the medicine
Not applicable.
Contraindications Never use MINOXIDIL SANDOZ 5%, solution for cutaneous application in the following cases:
Knownallergyto minoxidil or any of the components of the product,
scalplesions,
intoleranceto the 2% form,
subjectsunder 18 years of age or over 65 years of age,
anyalopecia (hair loss) in women.
IN CASE OF DOUBT, IT IS ESSENTIAL TO ASK YOUR DOCTOR OR PHARMACIST FOR ADVICE.
Precautions for use; special warnings
Take care with MINOXIDIL SANDOZ CONSEIL 5%, solution for cutaneous application:
Special warnings
In case of heart disease, even if it has existed for a long time, it is necessary to consult a doctor before using minoxidil for the first time.
In subjects with scalp lesions, an increase in the passage of the active ingredient into the blood is possible
In rare cases, or if the recommended dosage and/or method of administration is not followed, the passage of minoxidil into the systemic circulation may be increased, resulting in adverse effects such as chest pain, lowered blood pressure, tachycardia (increased heart rate), dizziness or lightheadedness, sudden unexplained weight gain, and swelling of the hands and feet If any of these symptoms occur, stop the treatment and consult your doctor.
The risk of hypertrichosis (abnormal development of the hair system) at a distance justifies not using this medicine in women.
Stop treatment and consult your doctor if redness or irritation of the scalp persists.
It is important to follow the recommended dosage and method of administration:
-donot increase the dose per application, do not increase the frequency of applications,
Only apply minoxidil to a healthy scalp. Avoid this application in case of irritation, persistent redness, inflammation, painful sensation of the scalp,
Donot apply minoxidil at the same time as retinoic acid or anthralin (drugs sometimes used in dermatology) or any other irritating dermatological drugs.
Do not apply minoxidil:
Incase of sudden hair loss, hair loss due to disease or drug treatment, DO NOT USE THIS MEDICATION AND TAKE THE ADVICE OF YOUR DOCTOR. Indeed, minoxidil would not be effective on this type of hair loss (see Health Education Tips).
onanother part of the body.
Precautions for use
This treatment requires regular medical monitoring, particularly at the beginning of treatment.
Do not swallow. Do not inhale.
In case of accidental contact with the eye, a wound or a mucous membrane, rinse thoroughly with running water.
A change in hair color and/or texture has been observed in some patients.
Sun exposure is not recommended when using minoxidil.
Tell your doctor if you have heart problems.
This medication contains propylene glycol and may cause skin irritation.
IF IN DOUBT, ASK YOUR DOCTOR OR PHARMACIST FOR ADVICE.
Interactions with other medicines
Taking or using other medications
Talk to your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
Interactions with food and drink
Not applicable.
Interactions with herbal products or alternative therapies
Not applicable.
Use during pregnancy and lactation
Pregnancy and lactation
This medicine is contraindicated in women.
Ask your doctor or pharmacist for advice before taking any medication.
Sportsmen
Not applicable.
Effects on ability to drive or use machines
Not applicable.
List of excipients with a known effect
List of excipients with a notable effect: propylene glycol.
3. HOW TO USE MINOXIDIL SANDOZ 5%, solution for cutaneous application
Instructions for proper use
Not applicable.
Dosage, Method and/or route(s) of administration, Frequency of administration and Duration of treatment
FOR ADULTS ONLY.
Dosage
Apply twice a day a dose of 1 ml on the scalp, starting from the centre of the area to be treated.
This dose must be respected regardless of the size of the area concerned.
The total daily dose should not exceed 2 ml.
Method of administration
Apply to the skin.
Spread the product with the fingertips so as to cover the entire area to be treated. Apply minoxidil only to a healthy scalp. Do not use it if you have irritation, persistent redness, inflammation or a painful scalp sensation.
Wash hands thoroughly after use.
Apply to perfectly dry hair and scalp.
Do not apply to any other part of the body.
The method of application varies depending on the delivery system used with the bottle.
Sprayer: This system is suitable for application to large areas of the scalp.
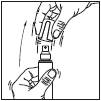

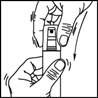
1. Remove the cap from the bottle.
2. Point the pump at the centre of the area to be treated, squeeze once and spread the product with your fingertips to cover the entire area to be treated. Repeat 6 times to apply a 1 ml dose (7 sprays in total).
Avoid inhaling the product.
3. Replace the cap on the bottle after use.
Sprayer with applicator: This system is suitable for application on small surfaces or under the hair.
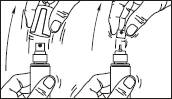
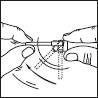
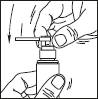
1. Remove the cap of the bottle.
2. Remove the top part of the pump. Lift up the angled tip of the applicator. Adjust the applicator and press firmly.
Then proceed as described for the sprayer:
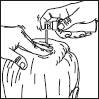
3. Point the wand towards the centre of the area to be treated, squeeze once and spread the product with the fingertips so that the entire area to be treated is covered. Repeat the operation 6 times to apply a dose of 1 ml (7 sprays in total).
Avoid inhaling the product.
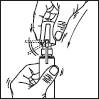
4. Fold down the applicator tip. Replace the cap on the bottle after use.
Frequency of administration
One application of 1 ml in the morning and 1 ml in the evening.
Do not increase the dose per application, do not increase the frequency of applications
Duration of treatment
A treatment of 2 months at a rate of 2 applications per day may be necessary before a stimulation of hair growth is demonstrated.
The onset and degree of response will vary according to the individual.
Do not discontinue applications as there is a risk of returning to the previous state after 3 or 4 months.
Symptoms and instructions in case of overdose
Not applicable.
Instructions in case of missed dose(s)
Not applicable.
Risk of withdrawal syndrome
Not applicable.
4. WHAT ARE THE POSSIBLE SIDE EFFECTS?
Description of side effects
Like all medicines, MINOXIDIL SANDOZ CONSEIL 5%, solution for cutaneous application is likely to have undesirable effects, although not everyone is subject to them.
Local irritation with desquamation (removal of dead skin cells), itching, erythema (redness of the skin), dry skin, acne, burning and hypertrichosis (abnormal growth of the hair system at a distance) or, more rarely, allergic reactions may occur. Breathing difficulties have also been reported. Your doctor should be informed.
In some rare cases, a drop in blood pressure, rapid pulse, palpitations, hair loss, irregular hair growth, chest pain, hepatitis or kidney stones may occur: stop the treatment immediately and inform your doctor.
More rarely, other reactions have been described, such as allergy (rhinitis, skin rash, generalized redness, facial swelling), dizziness, tingling, headache, weakness, edema (fluid in the tissues), taste alteration, ear infection, vision problems, eye irritation, neuritis (nerve damage)
It should be noted, however, that these effects, especially those that have been reported most rarely, have been reported without it being possible to formally establish that they were caused by the treatment.
Due to the presence of propylene glycol, risk of eczema,
Due to the presence of ethanol, frequent application to the skin may cause skin irritation and dryness,
Reporting side effects
If you experience any side effects, talk to your doctor or pharmacist. This also applies to any side effect not mentioned in this leaflet. You can also report adverse reactions directly via the national reporting system Agence nationale de sécurité du médicament et des produits de santé (Ansm) and the network of Regional Pharmacovigilance Centres. By reporting adverse reactions, you contribute to providing more information about the safety of the medicine.
5. HOW TO USE MINOXIDIL SANDOZ 5%, solution for cutaneous application
Keep out of the reach and sight of children.
Expiry date
Do not use MINOXIDIL SANDOZ CONSEIL 5%, solution for cutaneous application after the expiry date shown on the bottle and outer packaging. The expiry date refers to the last day of the month.
Storage conditions
Flammable product.
This medicine must be stored at a temperature below 25°C.
If necessary, warnings against certain visible signs of deterioration
Medicines should not be disposed of down the drain or in the household waste. Ask your pharmacist what to do with unused medicines. This will help protect the environment.
6. ADDITIONAL INFORMATION
Full list of active ingredients and excipients
What does MINOXIDIL SANDOZ CONSEIL 5%, solution for cutaneous application, contain?
The active substance is:
Minoxidil ................................................................................................................................................ 5 g
For 100 ml of solution.
The other ingredients are:
Propylene glycol, 96% ethanol, purified water.
Pharmaceutical form and content
What is MINOXIDIL SANDOZ CONSEIL 5%, solution for cutaneous application and what does it contain?
This medicine is presented as a solution for cutaneous application in a bottle.
Box of 1 or 3 vials.
Not all presentations may be marketed.
Name and address of the marketing authorisation holder and of the manufacturing authorisation holder responsible for batch release, if different
Holder
SANDOZ
49, avenue Georges Pompidou
92300 Levallois-Perret
 Français
Français English
English