FLECT'EXPERT CONCENTRATED MASSAGE BALM 30ML
Flect'Expert Massage Balm is used in case of muscular pains to relax stiff or tense muscles.
Free delivery for orders over 89€*.
* in metropolitan France and excluding drugs

Short-term local treatment for adults and children over 15, in case of minor trauma: sprains, contusions.
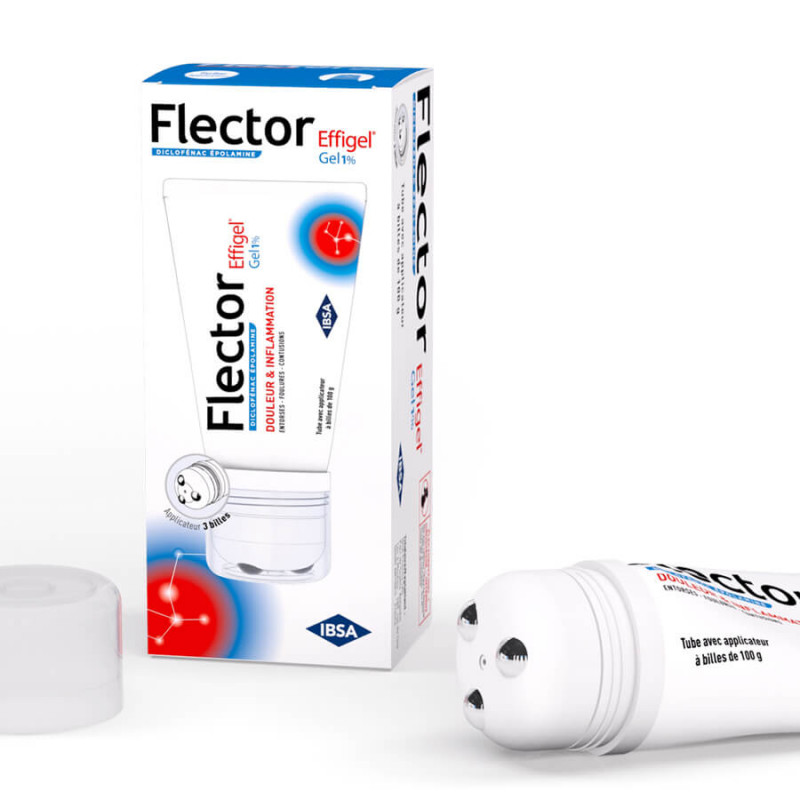
IBSA Flector Effigel 1% Gel Tube with Ball Applicator 100 gr is a short-term local treatment for adults and children over 15, in case of minor trauma: sprains, contusions.
Dosage: 1 application, 3 times a day. Gently massage the gel into the painful or inflammatory area.
Wash hands thoroughly after each use.
Duration of treatment : Treatment duration is limited to 4 days.
Method of administration : Local use - For adults and children over 15 years of age only.
EXTERNAL USE
Diclofenac epolamine 1.293 g
Corresponding amount of diclofenac sodium 1,000 g
Per 100 g gel
Notable excipients :
polyoxyethylenated hydrogenated castor oil, soy lecithin (2.4 g per 100 g gel), and Floral PH fragrance containing methyl benzoate, hydroxycitronellal, cinnamic alcohol, amyl cinnamal and benzyl salicylate.
Precautions for use
This drug is contraindicated in the following cases:
- hypersensitivity to the active substance or to any of the excipients listed in section 6.1.
- pregnancy, from the beginning of the 6th month (beyond 24 weeks of amenorrhea) (see section 4.6),
- damaged skin, whatever the lesion: oozing dermatitis, eczema, infected lesions, burns or wounds.
- Use in patients allergic to peanuts or soy is contraindicated.
Special warnings and precautions for use
- do not apply to mucous membranes or eyes; apply only to the painful area;
- if a rash appears after application, discontinue treatment immediately;
- This medicine contains castor oil and may cause skin reactions.
- This product contains methyl benzoate, which may cause local irritation.
- This medicine contains a fragrance containing the following allergens which may cause allergic reactions: hydroxycitronellal, cinnamic alcohol, amyl cinnamal, methyl benzoate and benzyl salicylate.
- This medicine must not be used under occlusive dressings.
Fertility, pregnancy and breast-feeding
Pregnancy
Inhibition of prostaglandin synthesis by NSAIDs may affect the course of pregnancy and/or the development of the embryo or fetus.
Risks associated with 1st trimester use
Data from epidemiological studies suggest an increased risk of miscarriage, cardiac malformations and gastroschisis, following treatment with a prostaglandin synthesis inhibitor in early pregnancy. The absolute risk of cardiovascular malformation increased from less than 1% in the general population, to approximately 1.5% in people exposed to NSAIDs. The risk appears to increase with dose and duration of treatment. In animals, administration of a prostaglandin synthesis inhibitor has been shown to result in increased pre- and post-implantation loss and embryo-fetal lethality. In addition, a higher incidence of certain malformations, including cardiovascular, has been reported in animals given a prostaglandin synthesis inhibitor during the organogenesis phase of gestation.
Risks associated with use from 12 weeks of amenorrhea to birth:
- From the 12th week of amenorrhea until birth, all NSAIDs, by inhibiting prostaglandin synthesis, can expose the fetus to renal impairment:
o in utero, as early as 12 weeks' amenorrhea (start of fetal diuresis): oligohydramnios (usually reversible on discontinuation of treatment), or even anammnios, particularly with prolonged exposure.
o at birth, renal insufficiency (reversible or not) may persist, particularly in cases of late and prolonged exposure (with a risk of delayed severe hyperkalemia).
Risks associated with use beyond the 24th week of amenorrhea and up to birth:
Beyond the 24th week of amenorrhea, NSAIDs can expose the fetus to cardiopulmonary toxicity (premature closure of the ductus arteriosus and pulmonary hypertension). Constriction of the ductus arteriosus can occur from the beginning of the 6th month (beyond the 24th week of amenorrhea) and can lead to fetal or neonatal right heart failure, or even fetal death in utero. This risk is all the greater the closer to term the dose is taken (less reversibility). This effect exists even when a single dose is taken.
At the end of pregnancy, both mother and newborn may experience :
- prolonged bleeding time, due to an anti-aggregation action which may occur even after very low doses of the drug have been administered;
- inhibition of uterine contractions, leading to delayed term or prolonged delivery.
Consequently:
Unless absolutely necessary, this drug should not be prescribed to women contemplating pregnancy or during the first 5 months of pregnancy (first 24 weeks of amenorrhea). If this drug is administered to a woman wishing to become pregnant or who is less than 6 months pregnant, the dose should be as low as possible and the duration of treatment as short as possible. Prolonged use is strongly discouraged.
From the beginning of the 6th month (beyond 24 weeks of amenorrhea): any intake of this drug, even punctual, is contraindicated. Inadvertent use after this date warrants cardiac, renal, fetal and/or neonatal monitoring, depending on the term of exposure. The duration of this monitoring should be adapted to the compound's elimination half-life
Breast-feeding
As A.I.N.S. is excreted in breast milk, this drug is not recommended for nursing mothers.
In the event of breast-feeding, this drug should never be applied to the breasts.
Fertility
As with all NSAIDs, the use of this drug may temporarily impair female fertility by affecting ovulation; it is therefore not recommended for women wishing to conceive a child. In women experiencing difficulties conceiving, or undergoing fertility tests, discontinuation of treatment should be considered.
Effects on ability to drive and use machines
Although the occurrence of such effects is highly unlikely when using skin preparations such as FLECTOREFFIGEL, patients who have previously experienced dizziness or other central nervous system disorders while taking NSAIDs should refrain from driving vehicles or operating machinery.
Flect'Expert Massage Balm is used in case of muscular pains to relax stiff or tense muscles.
Bioderma Cicabio Arnica+ 40 ml is a care formulated to attenuate the feelings of cutaneous discomfort in the adults, children and infants (except premature babies).
Indication: Care of the blows, blue, bumps.
Indications: This medicine is recommended for adults and children over 7 years of age as an adjunct to the treatment of minor trauma (bruises, blows) or insect bites.
The Bleus & Bosses Roll-on helps to soothe the pain by the cold effect of its formula. It contains organic essential oils of Italian Helichrysum and Peppermint and organic Arnica extract.
Practical to use and easy to carry
Homéoplasmine is used as an adjunct treatment for skin irritations.
Indications : This ointment contains extracts of plants and an antiseptic. It is used to soothe skin irritations.
Adults and children
Pharmacotherapeutic class: skin protector (D: Dermatology)
Also available in 40 gr. tube Homéoplasmine
Indications: Repairing and antibacterial complex - Irritated sensitive skin.
Infants - Children - Adults. Face and body
Red Tiger Balm is indicated for inflammations, muscular pains, osteoarticular, lumbar weakness and back pain.
The Syntholkiné spray is particularly adapted in first aid to relieve the small traumatisms. It is suitable for joint and muscle pain. Practical and quick to use. The Syntholkiné cold spray provides a cold effect that immediately relieves pain
Indications: This medicine is recommended for adults and children over 7 years of age as an adjunct to the treatment of minor trauma (bruises, blows) or insect bites.
Combined effectiveness: COLD + ESSENTIAL OILS
-Traumatic
-Muscular and articular
-Arthritic and arthritic
Homéoplasmine is used as an adjunct treatment for skin irritations.
Indications : This ointment contains extracts of plants and an antiseptic. It is used to soothe skin irritations.
Adults and children
Pharmacotherapeutic class: skin protector (D: Dermatology)
Also available in Homeoplasmine 18 gr tube
Vita Citral Soin TR+ Soothing Repair Gel 75 ml is a soothing repair gel for damaged and chapped hands.
Climatic or professional aggressions damage the hands and can be the cause of cracks and chapping. Vitacitral restores the barrier function, soothes and softens the skin.
Indication : Soothing repairing gel for damaged and chapped hands.
Indications: Repairing and antibacterial complex - Irritated sensitive skin.
Infants - Children - Adults. Face and body
Indications: Bariéderm Cica-Repairing Cream with Cu-Zn is the ideal care for small daily injuries of the whole family.
Indications: This medicine is a local antiseptic.
It is used forantisepsis of the skin (before surgery), shallow wounds and in the adjunctive treatment of skin lesions, infected or at risk of infection.