Naabak eye drops 4.9% Allergic eye diseases Bottle 10 ml
Eye drops in solution recommended to relieve the ocular symptoms of allergic origin (conjunctivites, blepharoconjunctivites).
Bottle of 10 ml of eye drops in solution.
Free delivery for orders over 89€*.
* in metropolitan France and excluding drugs

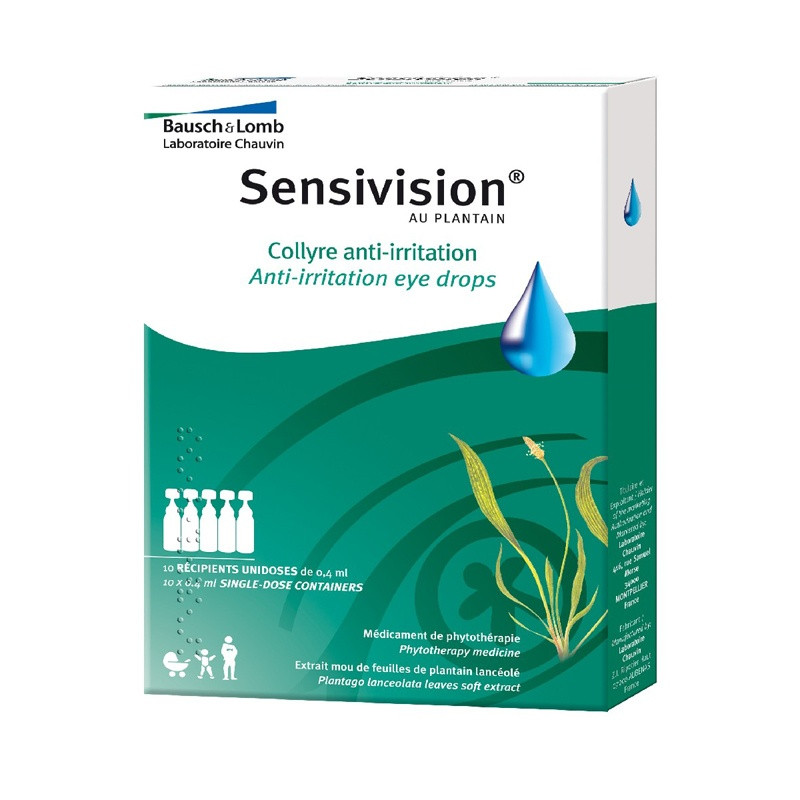
Indications: Traditionally used in case of irritation or ocular discomfort due to various causes (smoky atmosphere, sustained visual effort, sea or pool bathing, etc.).
In this manual :
1. WHAT IS PLANTAIN SENSIVISION, eye drops in single-dose containers AND WHAT IS IT USED FOR?
2. WHAT YOU SHOULD KNOW BEFORE USING SENSIVISION AU PLANTAIN, eye drops in single-dose containers
3. HOW TO USE SENSIVISION AU PLANTAIN, eye drops in single-dose containers?
4. WHAT ARE THE POSSIBLE SIDE EFFECTS?
5. HOW TO USE SENSIVISION WITH PLANT, eye drops in single-dose containers?
6. ADDITIONAL INFORMATION
1. WHAT IS PLANT SENSIVISION, eye drops in single-dose containers AND WHAT IS IT USED FOR?
HERBAL MEDICINAL PRODUCT.
Traditionally used in cases of irritation or eye discomfort due to various causes (smoky atmosphere, sustained visual effort, sea or pool bathing, etc.).
2. WHAT YOU SHOULD KNOW BEFORE USING PLANTAIN SENSIVISION, eye drops in single-dose containers
Not applicable.
Never use SENSIVISION AU PLANTAIN, eye drops in single dose containers in the following cases:
- allergy to any of the constituents.
Be careful with SENSIVISION AU PLANTAIN, eye drops in single-dose containers:
Special warnings
Reserve for minor ailments, if symptoms increase or persist for more than two days, consult a physician.
DO NOT USE:
- when irritation is accompanied by pus (eyelids stuck in the morning when waking up),
- in case of sharp pain, direct shock or injury.
Consult a doctor immediately.
Precautions for use
It is not advisable to wear contact lenses when using the eye drops. Wait 30 minutes before putting the lens back on the eye.
Taking or using other medicines
Talk to your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
Not applicable.
Not applicable.
Pregnancy and breastfeeding
Ask your doctor or pharmacist for advice before taking any medicine.
Not applicable.
Not applicable.
List of excipients with a notable effect: boric acid, borax, methyl parahydroxybenzoate.
3. HOW TO USE PLANTAIN SENSIVISION, single-dose eye drops
Not applicable.
Dosage, Method and/or route(s) of administration, Frequency of administration and Duration of treatment
Dosage
Instill 1 or 2 drops in each eye, 2 to 4 times a day.
Method of administration
Ophthalmic use.
Avoid contact of the tip of the container with the eye or fingers.
Single-use container: DO NOT REUSE.
Not applicable.
Not applicable.
Not applicable.
4. WHAT ARE THE POSSIBLE SIDE EFFECTS?
Like all medicines, PLANTAIN SENSIVISION Ophthalmic Solution in single-dose containers may have side effects, although not everyone is likely to have them.
If you notice any side effects not listed in this leaflet, or if any side effects become serious, please tell your doctor or pharmacist.
5. HOW TO STORE PLANTAIN SENSIVISION, eye drops in single dose containers
Keep out of the reach and sight of children.
Do not use SENSIVISION AU PLANTAIN, eye drops in single-dose containers after the expiry date stated on the box.
Store at a temperature below 25°C.
Do not store after opening the bottle.
Medicines should not be disposed of down the drain or in the household garbage. Ask your pharmacist what to do with unused medicines. This will help to protect the environment.
What does SENSIVISION WITH PLANTAIN contain?
The active substance is:
Lancet plantain (soft aqueous leaf extract) ................................................................................... 8 mg
For a single-dose container of 0.4 ml.
The other components are:
Boric acid, borax, disodium edetate, methyl parahydroxybenzoate, purified water.
What is SENSIVISION AU PLANTAIN, eye drops in single-dose containers and what does it contain?
This medicine is presented as an eye drop in a single-dose container. Box of 5, 10, 15 or 20.
Name and address of the marketing authorisation holder and of the manufacturing authorisation holder responsible for batch release, if different
LABORATOIRE CHAUVIN
416, RUE SAMUEL MORSE
CS99535
34961 MONTPELLIER CEDEX 2
LABORATOIRES CHAUVIN
LE MILLENAIRE B.P. 1174
34009 MONTPELLIER CEDEX I
LABORATOIRES CHAUVIN
ZI RIPOTIER HAUT
07200 AUBENAS
Not applicable.
The last date on which this package insert was approved was {date}.
Not applicable.
Detailed information on this medicine is available on the Afssaps (France) website.
Eye drops in solution recommended to relieve the ocular symptoms of allergic origin (conjunctivites, blepharoconjunctivites).
Bottle of 10 ml of eye drops in solution.
Helps the proper functioning of the muscles.
Stretching, twisting of the muscles... are the signs of involuntary muscle contractions.
Most of the time, this type of painful and brutal contractions occur at rest (especially at night) or following a physical exercise
One of the main causes of these involuntary contractions is an imbalance of trace elements, vitamins and minerals in our body.
Indications : Herbal medicine. Rhodiola is known to promote intellectual tonus and to increase resistance to stress.
SENSIOPTIC SPRAY LUBRIFICATION EYES and EYES: a formula with SOOTHING Myritol and HYDRATING hyaluronic acid.
Timoferol contains iron and is indicated for the curative treatment of iron deficiency anemia in adults and children over 12 years of age, and for the preventive treatment of iron deficiency during pregnancy.
Indications: Known for its draining virtues, the heather is used to support the urinary comfort. Moreover, it contributes to the elimination of water.
Bronchokod sugar-free syrup contains 5% carbocistein. This non-prescription medicine is a fluidifier of bronchial secretions, and thus facilitates their evacuation by coughing.
Immuvit' 4G supports immunity and activates vitality thanks to its complete and technical formula composed of vitamins, minerals, ferments and plants.
- Triple release tablet
- Reinforced with eleutherococcus, vitamin D and zinc
Rocgel, drinkable suspension in sachet-dose is an antacid. Indeed, this medicine is recommended in pain, heartburn or burns of the stomach or esophagus
Urgo Hand & Foot Warts Cryotherapy Treatment freezes warts on the hands and feet in one treatment.
Designed to relieve intense and persistent dry eye, as well as abnormal tearing caused by conditions such as chronic blepharitis or eye surgery. This product is ideal for people suffering from severe dry eye.
Its cross-linked hyaluronic acid formula provides immediate and long-lasting comfort, while preserving ocular health.
Compatible with contact lenses, they are suitable for frequent use.
Available in 10 Unidose and 10 ml Bottle formats
Gelox, drinkable suspension in sachet is a medicine for adults, recommended in pain, heartburn or heartburn of the stomach or esophagus
Mycoster 1% powder for dermal application is a medication recommended for the treatment of inter-toe mycosis caused by dermatophytes (a variety of fungus between the toes).
This topical antifungal agent is not indicated for the treatment of other folds
Discover the Somnifor gummies from Santarome that help you fall asleep and reduce nighttime awakenings.
Somnifor Gummies allows to accelerate the falling asleep. To decrease the night awakenings. Have a restful sleep.
It is addressed to the people wishing to sleep better and to have a repairing sleep.
Key ingredients: Melatonin, Hawthorn, Lime and Fig buds, California Poppy
Duration of the cure: 1 month.
Taste: Fruits of the wood
- Adhesive devices that allow your child to breathe freely with eucalyptus vapors.
This Anti-Hair Loss and Growth serum has been specifically tested as a companion product to the leading topical drug treatments on areas harvested and implanted for hair transplantation. It can be used alone or in combination with a local drug treatment.
Application on dry or wet scalp
8 sprays / Daily use