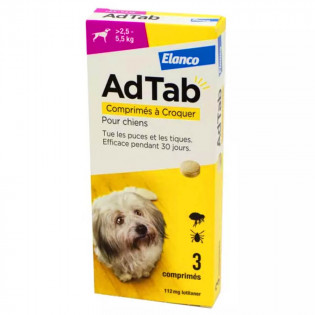
AdTab Chewable tablets for dogs from 2.5 to 5.5 kg 3 tablets provide immediate and persistent insecticidal activity against fleas (Ctenocephalus felis and Ctenocephalus canis) and acaricidal activity against ticks (Rhipicephalus sanguineus, Ixodes ricinus, Ixodes hexagonus and Dermacentor reticulatus) for 1 month.
Fleas and ticks must be attached to the host and have begun their meal to be exposed to the active substance, lotilaner.
Each chewable tablet contains 112.5 mg lotilaner.
ADTAB kills newly hatched fleas on the dog before they lay their eggs. As a result, ADTAB breaks the flea life cycle and prevents environmental contamination by fleas in areas accessible to the dog.
Lotilaner :
Lotilaner, a pure enantiomer belonging to the isoxazoline class, is effective against both fleas and ticks.
It is a powerful inhibitor of the chloride channels of gamma-aminobutyric acid (GABA) receptors, causing the rapid death of ticks and fleas.
The activity of lotilaner was not affected by resistance to organochlorines (cyclodienes e.g. dieldrin), phenylpyrazoles (e.g. fipronil), neonicotinoids (e.g. imidacloprid), formamidines (e.g. amitraz) and pyrethroids (e.g. cypermethrin).
For fleas, the product is effective within 4 hours of attachment, for 1 month after administration. Fleas present on the animal before administration of the product are killed within 6 hours.
For ticks, the product is effective within 48 hours of attachment, for 1 month after administration. Ixodes ricinus ticks present on the animal before administration of the product are killed within 8 hours.
Directions for use AdTab Chewable tablets for dogs from 2.5 to 5.5 kg 3 tablets
For optimum control of flea and tick infestation, the product should be administered monthly throughout the flea and/or tick season, depending on the local epidemiological situation.
Administer 1 palatable chewable tablet every month, during or after a meal.
Dose of 20 to 43 mg lotilaner per kg bodyweight.
Wash hands after handling.
Please note
To ensure correct dosage, body weight should be determined as accurately as possible.
Underdosing could result in a lack of efficacy and encourage the development of resistance.
Precautions for use :
Veterinary use.
See oral.
Keep out of reach and sight of children.
Special precautions for safe use in target species:
Parasites must have begun feeding on the host before being exposed to lotilaner; consequently, the risk of transmission of infectious diseases of parasitic origin cannot be excluded.
The possibility that other animals in the same household could be a source of flea reinfestation must be taken into account, and these should be treated if necessary with an appropriate product.
Fleas are capable of infesting dog bedding and regular resting areas such as carpets and upholstery during all stages of their development. In the event of mass flea infestation, these areas must be treated with a suitable environmental product, then vacuumed regularly from the outset.
The safety and efficacy of the product have not been evaluated on puppies under 8 weeks of age or dogs weighing less than 1.3 kg.
In the absence of available data, veterinary advice should be sought before administering this product to puppies under 8 weeks of age or dogs weighing less than 1.3 kg.
Special precautions to be taken by the person administering the veterinary drug to animals:
Wash hands after handling product.
In the event of accidental ingestion, seek medical advice immediately and show the leaflet or label.
Pregnancy and lactation :
Laboratory studies in rats have shown no evidence of teratogenic effects. The safety of the drug during pregnancy and lactation has not been established.
Consult a veterinarian prior to treatment during gestation and lactation.
Fertility :
Laboratory studies in rats and rabbits have shown no adverse effects on male or female reproductive performance.
Safety in breeding dogs has not been established.
Drug and other interactions :
None known.
In clinical trials, no interactions were observed between AdTab and commonly used veterinary drugs.
Overdose (symptoms, emergency treatment, antidotes):
No adverse effects were observed after oral administration in puppies aged 8 to 9 weeks, weighing 1.3 to 3.6 kg, and treated up to 5 times the maximum recommended dose (43 mg, 129 mg and 215 mg lotilaner/kg body weight) on eight occasions, at monthly intervals.
Adverse reactions :
Very rare (< 1 animal / 10000 treated animals, including isolated cases :
Diarrhea (1) (2).
Vomiting (1) (2).
Anorexia (1) (2).
Lethargy (2).
Ataxia (3).
Convulsion (3).
Tremor (3).
It is important to report adverse reactions. Reporting allows continuous monitoring of drug safety.
If you notice any adverse effects, even those not listed in the package leaflet, or if you think the drug has not been effective, please contact your veterinarian in the first instance. You can also report any adverse reaction to the marketing authorization holder using the contact details on the package leaflet or via the national reporting system: https://pharmacovigilance-anmv.anses.fr/.
(1) mild and transient.
(2) generally disappear without treatment.
(3) transient in most cases.
AdTab Chewable tablets for dogs from 2.5 to 5.5 kg 3 tablets provide immediate and persistent insecticidal activity against fleas (Ctenocephalus felis and Ctenocephalus canis) and acaricidal activity against ticks (Rhipicephalus sanguineus, Ixodes ricinus, Ixodes hexagonus and Dermacentor reticulatus) for 1 month.
Fleas and ticks must be attached to the host and have begun their meal to be exposed to the active substance, lotilaner.
Each chewable tablet contains 112.5 mg lotilaner.
ADTAB kills newly hatched fleas on the dog before they lay their eggs. As a result, ADTAB breaks the flea life cycle and prevents environmental contamination by fleas in areas accessible to the dog.
Lotilaner :
Lotilaner, a pure enantiomer belonging to the isoxazoline class, is effective against both fleas and ticks.
It is a powerful inhibitor of the chloride channels of gamma-aminobutyric acid (GABA) receptors, causing the rapid death of ticks and fleas.
The activity of lotilaner was not affected by resistance to organochlorines (cyclodienes e.g. dieldrin), phenylpyrazoles (e.g. fipronil), neonicotinoids (e.g. imidacloprid), formamidines (e.g. amitraz) and pyrethroids (e.g. cypermethrin).
For fleas, the product is effective within 4 hours of attachment, for 1 month after administration. Fleas present on the animal before administration of the product are killed within 6 hours.
For ticks, the product is effective within 48 hours of attachment, for 1 month after administration. Ixodes ricinus ticks present on the animal before administration of the product are killed within 8 hours.
Directions for use AdTab Chewable tablets for dogs from 2.5 to 5.5 kg 3 tablets
For optimum control of flea and tick infestation, the product should be administered monthly throughout the flea and/or tick season, depending on the local epidemiological situation.
Administer 1 palatable chewable tablet every month, during or after a meal.
Dose of 20 to 43 mg lotilaner per kg bodyweight.
Wash hands after handling.
Please note
To ensure correct dosage, body weight should be determined as accurately as possible.
Underdosing could result in a lack of efficacy and encourage the development of resistance.
Precautions for use :
Veterinary use.
See oral.
Keep out of reach and sight of children.
Special precautions for safe use in target species:
Parasites must have begun feeding on the host before being exposed to lotilaner; consequently, the risk of transmission of infectious diseases of parasitic origin cannot be excluded.
The possibility that other animals in the same household could be a source of flea reinfestation must be taken into account, and these should be treated if necessary with an appropriate product.
Fleas are capable of infesting dog bedding and regular resting areas such as carpets and upholstery during all stages of their development. In the event of mass flea infestation, these areas must be treated with a suitable environmental product, then vacuumed regularly from the outset.
The safety and efficacy of the product have not been evaluated on puppies under 8 weeks of age or dogs weighing less than 1.3 kg.
In the absence of available data, veterinary advice should be sought before administering this product to puppies under 8 weeks of age or dogs weighing less than 1.3 kg.
Special precautions to be taken by the person administering the veterinary drug to animals:
Wash hands after handling product.
In the event of accidental ingestion, seek medical advice immediately and show the leaflet or label.
Gestation and lactation :
Laboratory studies in rats have shown no evidence of teratogenic effects. The safety of the drug during pregnancy and lactation has not been established.
Consult a veterinarian prior to treatment during gestation and lactation.
Fertility :
Laboratory studies in rats and rabbits have shown no adverse effects on male or female reproductive performance.
Safety in breeding dogs has not been established.
Drug and other interactions :
None known.
In clinical trials, no interactions were observed between AdTab and commonly used veterinary drugs.
Overdose (symptoms, emergency treatment, antidotes):
No adverse effects were observed after oral administration in puppies aged 8 to 9 weeks, weighing 1.3 to 3.6 kg, and treated up to 5 times the maximum recommended dose (43 mg, 129 mg and 215 mg lotilaner/kg body weight) on eight occasions, at monthly intervals.
Adverse reactions :
Very rare (< 1 animal / 10000 treated animals, including isolated cases :
Diarrhea (1) (2).
Vomiting (1) (2).
Anorexia (1) (2).
Lethargy (2).
Ataxia (3).
Convulsion (3).
Tremor (3).
It is important to report adverse reactions. Reporting allows continuous monitoring of drug safety.
If you notice any adverse effects, even those not listed in the package leaflet, or if you think the drug has not been effective, please contact your veterinarian in the first instance. You can also report any adverse reaction to the marketing authorization holder using the contact details on the package leaflet or via the national reporting system: https://pharmacovigilance-anmv.anses.fr/.
(1) mild and transient.
(2) generally disappear without treatment.
(3) transient in most cases.

 Français
Français English
English