PAPULEX OIL FREE CREAM FOR BLEMISHED SKIN 40ML
Description: Papulex Oil-Free Cream improves the condition of your skin by helping to clear up imperfections. Recommended for problem skin.
Free delivery for orders over 89€*.
* in metropolitan France and excluding drugs

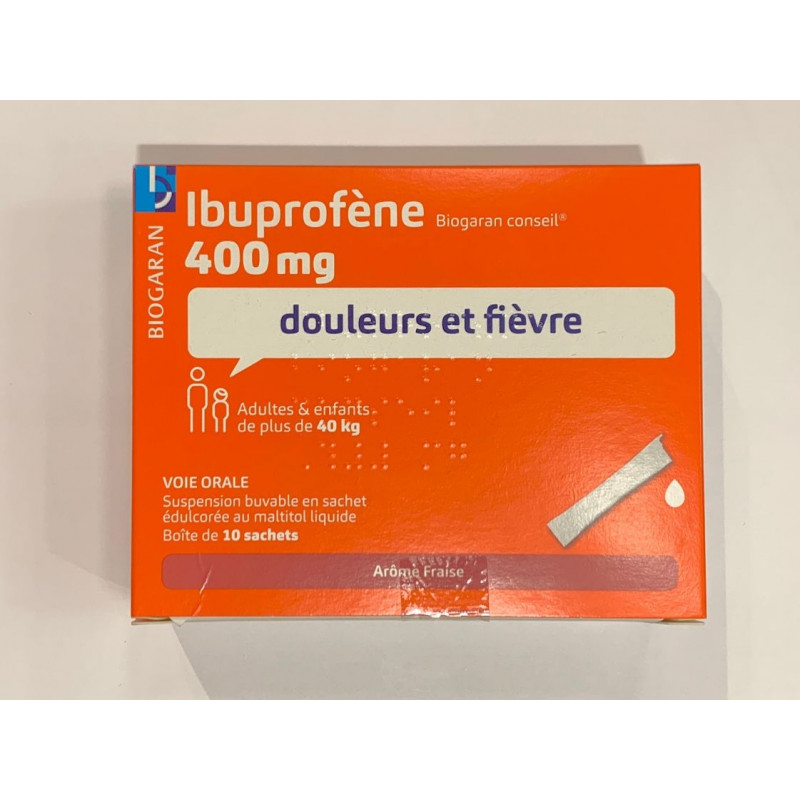
Drinkable suspension in sachet sweetened with liquid maltitol.
Strawberry flavour. Adults and children (over 12 years) weighing more than 40 kg.
Pain and fever.
This medicine contains a non-steroidal anti-inflammatory drug: ibuprofen. It is indicated for adults and children weighing more than 30 kg (approximately 11-12 years) for the short-term treatment of fever and/or pain such as headaches, flu, toothache, aches and pains, painful periods.
Suitable for adults and children weighing over 30 kg (11-12 years).
Painful and/or feverish conditions:
The usual dosage is 1 tablet of 400 mg per dose, to be repeated if necessary, without exceeding 3 tablets of 400 mg per day (i.e. 1200 mg per day).
The 400 mg tablet is reserved for pain or fever that is more intense or not relieved by a 200 mg ibuprofen tablet.
As the elderly are at increased risk of adverse effects, use the lowest possible dose for the shortest time necessary to relieve symptoms.
Do not exceed recommended doses or duration of treatment (3 days for fever, 5 days for pain).
The maximum dosage is 3 tablets per day (1200 mg).
Oral.
Swallow the tablet, without chewing, with a large glass of water.
The tablets should preferably be taken with a meal.
Systematic administration avoids fluctuations in pain or fever.
They should be taken at least 6 hours apart.
Duration of treatment
The duration of use is limited to:
If the pain persists for more than 5 days or the fever for more than 3 days, or if they worsen, or if another disorder occurs, inform your doctor.
If you feel that the effect of Ibuprofen Biogaran Conseil 400mg is too strong or too weak: consult your doctor or pharmacist.
If you are a woman, Ibuprofen Biogaran Conseil 400mg may affect your fertility. In women who have difficulty in conceiving or who are undergoing reproductive tests, please talk to your doctor or pharmacist before taking Ibuprofen Biogaran Conseil 400mg. Elderly patients have a higher risk of adverse reactions, especially gastrointestinal bleeding, ulcers and perforations. Renal, hepatic and cardiac function should be closely monitored. Dosage should be kept as low as possible for the shortest time necessary to relieve symptoms. Before using this medication, consult your physician if you have: A history of asthma associated with chronic rhinitis, chronic sinusitis or polyps in the nose. The administration of this speciality may lead to an asthma attack, especially in some people who are allergic to acetylsalicylic acid (aspirin) or to non-steroidal anti-inflammatory drugs. Coagulation disorders, taking an anticoagulant treatment. This drug may cause severe gastrointestinal manifestations. Digestive history (hiatal hernia, digestive hemorrhage, old stomach or duodenal ulcer). Heart, liver or kidney disease. Chicken pox. This medication is not recommended due to exceptional severe skin infections. Concomitant treatment with other drugs that increase the risk of peptic ulcer or bleeding, for example oral corticosteroids, antidepressants (those of the SSRI type, i.e. Selective Serotonin Reuptake Inhibitors), drugs that prevent blood clots such as aspirin, or anticoagulants such as warfarin If you have any of these conditions, consult your doctor before taking Ibuprofen Biogaran 400mg. Concomitant treatment with methotrexate at doses greater than 20 mg per week or with pemetrexed. During treatment, if you experience: Visual problems, tell your doctor. Gastrointestinal bleeding (blood in the mouth or stool, black stool), stop treatment and seek immediate medical attention. If any signs appear on the skin or mucous membranes that resemble a burn (redness with blisters or blisters, ulcerations), stop the treatment and contact a doctor or emergency medical service immediately. If there are any signs of allergy to this medicine, including an asthma attack or sudden swelling of the face and neck, stop the treatment and contact a doctor or emergency medical service immediately. This medicine contains a non-steroidal anti-inflammatory drug: ibuprofen. You should not take other medicines containing non-steroidal anti-inflammatory drugs (including selective cyclooxygenase 2 inhibitors) and/or acetylsalicylic acid (aspirin) with this medicine. Carefully read the package inserts of other medicines you are taking to make sure they do not contain non-steroidal anti-inflammatory drugs and/or acetylsalicylic acid (aspirin).
At high doses, above 1200 mg/day, this medication has anti-inflammatory properties and may cause sometimes serious inconveniences which are those observed with non-steroidal anti-inflammatory drugs.
Medicines such as Ibuprofen Biogaran Conseil 400mg could increase the risk of heart attack ("myocardial infarction") or stroke. The risk is greater the higher the doses used and the longer the duration of treatment.
Do not exceed the recommended doses or duration of treatment.
If you have heart problems, have had a stroke, or think you may have risk factors for these conditions (e.g. high blood pressure, diabetes, high cholesterol, or smoking), please talk to your doctor or pharmacist
During the first trimester of pregnancy (12 weeks of amenorrhea, i.e. 12 weeks after the first day of your last menstrual period), your doctor may prescribe this medicine if necessary.
From 2.5 to 5 months of pregnancy (12 to 24 weeks of amenorrhea), this medicine should only be used on the advice of your doctor and taken for short periods. Prolonged use of this medication is strongly discouraged.
If you are more than 5 months pregnant (more than 24 weeks of amenorrhea), you should not take this medicine under any circumstances, as its effects on your child may have serious consequences, particularly in terms of cardiopulmonary and renal health, even if you take it only once.
If you have taken this medicine when you are more than five months pregnant, please talk to your obstetrician so that you can be offered appropriate monitoring.
This medicine passes into breast milk. As a precautionary measure avoid using it while breastfeeding.
Never take Ibuprofen Biogaran Conseil 400mg in the following cases:
In case of doubt, it is essential to ask your doctor or pharmacist for advice.
Like all medicines, Ibuprofen Biogaran Conseil 400mg is likely to have side effects, although not everyone is subject to them.
Medicines such as Ibuprofen Biogaran Conseil 400mg may increase the risk of heart attack (myocardial infarction) or stroke.
Allergic reactions may occur:
In some rare cases, gastrointestinal bleeding may occur. The higher the dosage used, the more frequent it is.
Headache with nausea, vomiting and neck stiffness may exceptionally occur.
In exceptional cases, severe skin infections have been observed with chickenpox.
Very exceptionally, bullous manifestations of the skin or mucous membranes (burning sensation with redness and bullae, blisters, ulcerations) may occur.
In all these cases, treatment must be stopped immediately and your doctor informed.
During treatment, the following may occur
In all cases, you should inform your doctor.
Exceptionally, changes in the liver or blood count (decrease in white or red blood cells) have been observed, which may be serious.
If you notice any side effects not mentioned in this leaflet, or if any side effects become serious, please inform your doctor or pharmacist.
Active ingredients
Ibuprofen: 400 mg
For one film-coated tablet.
Pregelatinised starch, sodium carboxymethyl starch (type A), stearic acid, povidone K90, colloidal anhydrous silica.
Film coating: SEPIFILM white [hypromellose (E464), microcrystalline cellulose, macrogol 40 stearate (type I), titanium dioxide (E171)].
Description: Papulex Oil-Free Cream improves the condition of your skin by helping to clear up imperfections. Recommended for problem skin.
Phytoxil Cough and Throat is a medical device in the form of a syrup intended to reduce the symptoms of dry cough and to relieve throat irritation in adults and children, from 2 years of age.
Aurigoutte ear drops are indicated as an antiseptic for closed ear drum external otitis and as an analgesic for these
Septivon is a local antiseptic solution associated with a foaming agent. It works by destroying bacteria. It is used to clean or treat locally infected skin or skin exposed to a risk of infection.
Packaging: 250 ml or 500 ml bottle
Nervousness ,Mild sleep disorders - Adults and children
Borax/Boric acid of Mylan Laboratory is a solution for ophthalmic washing. This medicine is presented in the form of single-dose containers of 5 ml.
It is indicated for eye washing in case of conjunctival irritation.
Description: dry, tired, burning and watery eyes
MEDIFLOR
Oropolis
Soothes the throat
Propolis taste honey lemon
Sugar free
Food supplement
Soothing tablets x20
Indications: This medicine is indicated as a complement to rehydration and/or dietary measures, in the symptomatic treatment of diarrhoea in adults and children over 6 years.
Pruriced SOS After-Bites is a soothing gel in the form of roll-on, indicated to relieve the unpleasant feelings associated with the insect bites and plants.
- Treatment of allergies,
- From 6 years old.
Doxylamine is a hypnotic (sleeping pill) which contains an antihistamine with a sedative effect. It is therefore used to treat occasional insomnia in case of sleep disorders and falling asleep.
Warning: The delivery is limited to 6 boxes of Doxylamine per month, all brands included.
Description: Calming essential oil, dedicated to the respiratory sphere, anti-infectious, mucolytic, expectorant, analgesic, anti-inflammatory, local anesthetic, healing
White Tiger Balm is indicated against nasal congestion, sinusitis, rhinitis, coughs, colds, headaches and insect bites.
Erythema secondary to radiotherapy treatments.
First and second degree burns and all other non-infected skin wounds.
Fertifol 400 micrograms is a folic acid medicine, offered in tablet form. It helps to correct alterations in methionine metabolism. This medicine is also indicated for prevention of neural tube closure anomaly in case of pregnancy desire.