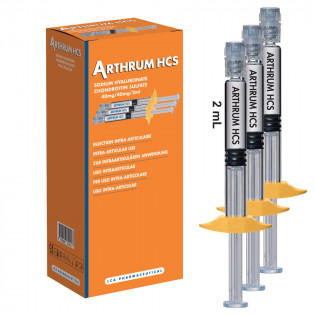
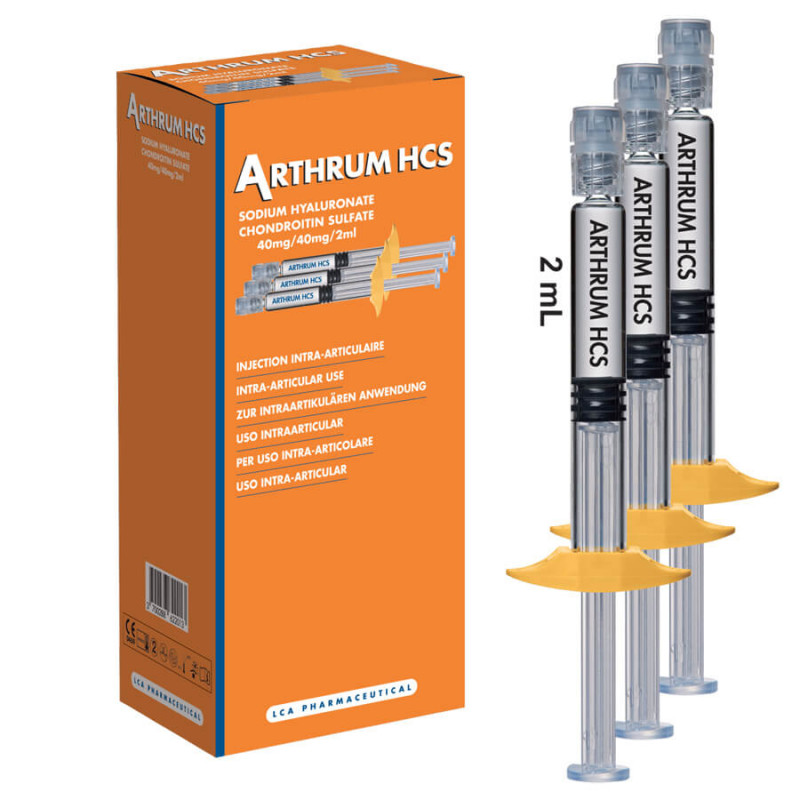
Description Arthrum HCS 40mg/2ml arthritis 3 syringes
ARTHRUM HCS is a sterile, injectable viscoelastic device containing two components:
- Sodium Hyaluronate is a glycosaminoglycan derived naturally from hyaluronic acid, obtained by bio-fermentation, with a high molecular weight of 2.8 million Daltons and high concentration (20mg/ml). Sodium Hyaluronate is naturally present in the extracellular matrix, in the soft connective tissues and in the synovial fluid where it is synthesized by the synoviocytes. Being viscoelastic, synovial fluid acts as a lubricant at low frequency, and as a shock absorber at high frequency, limiting mechanical stress and pain in the joint.
- Chondroitin Sulfate is a highly purified, high concentration 20mg/ml glycosaminoglycan of marine origin. Chondroitin Sulfate, naturally present in the connective tissue, is one of the major constituents of the cartilage matrix. Its function is to maintain osmotic pressure by absorbing water and contributing to the hydration of cartilage.
The components of ARTHRUM HCS are not chemically modified, thus eliminating any risk of allergy and potential cytotoxicity and guaranteeing safety and perfect tolerance.
Form and presentation Arthrum HCS 40mg/40mg/2ml osteoarthritis
ARTHRUM HCS is a sterile, transparent, homogeneous viscoelastic preparation made from highly purified injectable grades of Sodium Hyaluronate and Chondroitin Sulfate, in accordance with the monographs of the European Pharmacopoeia.
ARTHRUM HCS is a sterile solution with physiological pH, presented in a single-use glass syringe, equipped with a Luer Lock and pre-filled to 2 ml.
The syringe is labelled with the product designation. The syringe is contained in an individual sterility protector.
The syringe, the sterile intra-articular injection needle and the accessories (information leaflet, traceability labels) are included in a packaging case with all the identification details of the ARTHRUM HCS viscoelastic injection device.
ARTHRUM HCS is available in boxes of 1 and 3 syringes.
The pre-filled syringe of ARTHRUM HCS is sterilized by steam pressure in its individual sterility protector. The contents and exterior of the syringe are sterile, so that ARTHRUM HCS can be safely used in the operating room.
The contents of each syringe are sterile and non-pyrogenic.
Properties Arthrum HCS 40mg/40mg/2ml osteoarthritis 3 syringes
Sodium Hyaluronate is a glycosaminoglycan naturally present in humans and is a major constituent of the inter-cellular spaces. In the joints, it is a structural component of the cartilage and synovial fluid, which acts as a lubricant, shock absorber, filter, and metabolic intermediate.
Chondroitin Sulfate is also a glycosaminoglycan, an essential component of the cartilage matrix, to which it provides excellent resistance to compression.
ARTHRUM HCS is biologically similar to human synovial fluid and participates in the following functions
Protecting cartilage
- The lubricating properties of the Sodium Hyaluronate and Chondroitin Sulfate molecules contained in ARTHRUM HCS allow the joint surfaces to slide easily over each other, and protect them from mechanical damage.
- The elastic properties of the sodium hyaluronate molecules reduce stress under load, thus protecting the cartilage from excessive compressive forces.
- The high adhesion and covering capacity of Chondroitin Sulfate improves the protection of cartilage and other endo-articular tissues.
Protecting the synovial membrane
The normal synovial membrane lines the joint cavities. It ensures the trophicity of the articular cartilage and the secretion of synovial fluid. It constitutes a filtration and defense barrier.
Sodium Hyaluronate and Chondroitin Sulfate provide a protective barrier by masking the pain receptors present in the synovial membrane.
Reduce pain and improve mobility
- Sodium Hyaluronate and Chondroitin Sulfate replace and improve the viscoelasticity of pathological synovial fluid in osteoarthritic joints.
As a result, they help maintain intra-articular spaces, reduce pain and restore joint mobility.
Indications Arthrum HCS 40mg/40mg/2ml osteoarthritis 3 syringes
The therapeutic indications are all types of painful osteoarthritis of the synovial joints, in particular gonarthrosis.
Instructions for use and dosage Arthrum HCS 40mg/40mg/2ml
- Check the integrity of the individual sterility protector and the expiration date.
- Aseptically open the individual sterility protector.
- Aseptically pick up the syringe and injection needle.
- Carefully unscrew the stopper from the syringe tip.
- Screw the injection needle onto the Luer Lock system without touching the tip of the syringe.
- Proceed with the intra-articular injection.
- The syringe and needle should be disposed of immediately after use in a dedicated single-use waste container.
Dosage :
Inject ARTHRUM HCS into the affected joint once a week for 3 weeks.
PRECAUTIONS FOR USE:
The following precautions for use are recommended:
- Do not use if the individual sterility protector of the product is damaged.
- Respect the rules of asepsis.
- Do not inject other products at the same time as ARTHRUM HCS.
- Systematically aspirate any synovial fluid effusion beforehand to ensure intra-articular position.
- Precautions for use are those required by the protocol used for intra-articular injections in rheumatology. The specialist physician remains responsible for his or her own techniques and indications.
- It is recommended to inform patients, as for any intra-articular injection, to remain at rest for 24 hours and to avoid any sports or professional activity.
Warnings: The syringe should be used only once, the contents of the syringe should be used immediately after the individual safety shield is opened. Destroy the syringe if the product has not been used and the individual sterility protector has been opened.
Do not resterilize the product in the syringe by heat or any other process.
The risks are loss of physicochemical properties and microbiological contamination of the product, leading to a serious risk of infection and inflammation of the joint.
Do not inject intravascularly.
Do not inject outside the joint cavity or into the synovial tissue or capsule and or in the presence of
presence of a large effusion.
ARTHRUM HCS has not been studied in pregnant women.
It has not been established that ARTHRUM HCS can regenerate cartilage.
Incompatibilities :
Do not use quaternary ammonium (Benzalkonium Chloride) for skin disinfection when using ARTHRUM HCS.
Contra-indications:
- All inflammatory conditions of the joint that should be treated prior to treatment with intra-articular injection of ARTHRUM HCS.
- Do not administer if the patient has a known hypersensitivity to Sodium Hyaluronate and Chondroitin Sulfate.
- ARTHRUM HCS has not been tested in pregnant or nursing women, nor in children under 18 years of age.
Adverse reactions:
ARTHRUM HCS is well tolerated in men. Sometimes during 48 hours a slight pain and or a moderate edema can occur. It is recommended to apply an ice bladder for a few hours.
Composition:
One syringe (2ml) contains 40 mg of sodium Hyaluronate and
40 mg of Chondroitin Sulfate.
Conservation
Protect from light and frost. Store between +2°C and +25°C.
 Français
Français English
English