Arnica Cream LPMF 50 ml
Indications:
Cuts, bumps, bruises, contusions. Calms and soothes. Enriched with arnica extract and essential oil. From 3 years.
Free delivery for orders over 89€*.
* in metropolitan France and excluding drugs

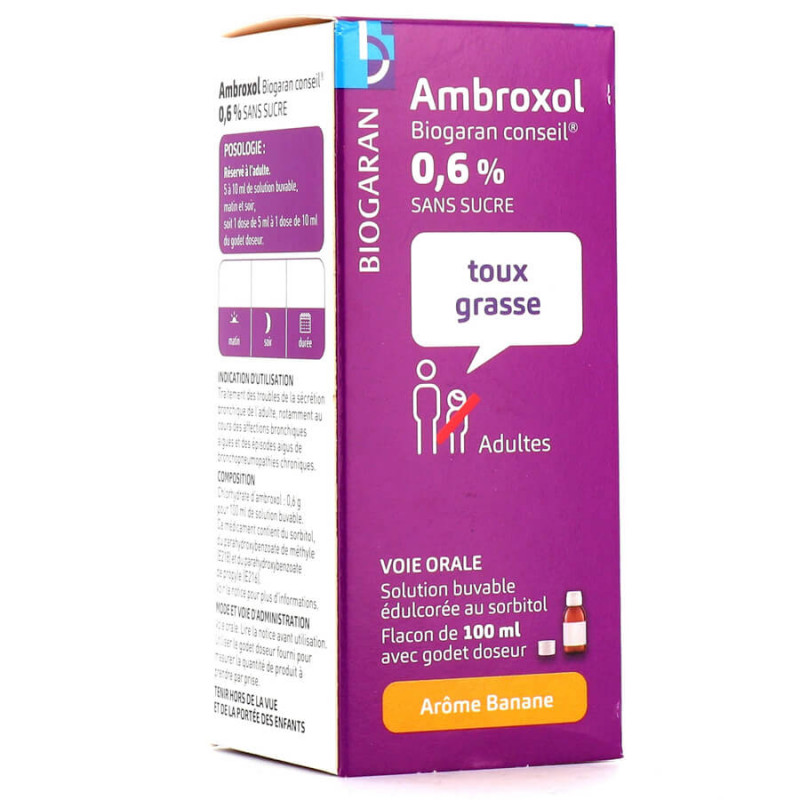
Indications: Fatty cough. Treatment of bronchial secretion disorders in adults, particularly during acute bronchial diseases.
1. NAME OF THE MEDICINAL PRODUCT
AMBROXOL BIOGARAN CONSEIL 0.6% SUGAR-FREE, drinkable solution sweetened with sorbitol
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Ambroxol hydrochloride ....................................................................................................................... 0,6 g
For 100 ml of drinkable solution.
Excipients: sorbitol, methyl parahydroxybenzoate (E218), propyl parahydroxybenzoate (E216).
For a complete list of excipients, see section 6.1.
Oral solution.
Treatment of bronchial secretion disorders in adults, particularly during acute bronchial diseases and acute episodes of chronic lung disease.
4.2. Dosage and method of administration
RESERVED FOR ADULTS.
5 ml of oral solution contains 30 mg of ambroxol hydrochloride.
The average dosage of ambroxol hydrochloride is 60 to 120 mg per day divided into 2 doses, i.e. 5 to 10 ml twice a day (morning and evening).
A measuring cup graduated at 2.5 ml, 5 ml, 7.5 ml, 10 ml and 15 ml or a 5 ml measuring spoon are provided to measure the quantity of active ingredient for one dose:
Either 5 ml to 10 ml from the measuring cup or 1 to 2 measuring spoons.
History of hypersensitivity reactions to any of the constituents.
4.4. Special warnings and precautions for use
The combination of a bronchial mucosifier with a cough suppressant and/or secretion drying substances (atropine) is not recommended.
This medicine contains methyl parahydroxybenzoate (E218) and propyl parahydroxybenzoate (E216) and may cause allergic reactions (possibly delayed).
This medicine contains sorbitol. Its use is not recommended in patients with fructose intolerance.
Due to the presence of sorbitol, this medicine may cause a moderate laxative effect.
4.5. Interactions with other medicinal products and other forms of interaction
Not applicable.
Pregnancy
Animal studies have not shown any evidence of a teratogenic effect. In the absence of a teratogenic effect in animals, a malformative effect in humans is not expected.
Indeed, to date, substances responsible for malformations in humans have been shown to be teratogenic in animals in well-conducted studies in two species.
Clinically, there are currently no sufficiently relevant data to assess a possible malformative or fetotoxic effect of ambroxol hydrochloride when administered during pregnancy.
Therefore, as a precautionary measure, ambroxol hydrochloride should not be used during pregnancy.
Breastfeeding
The use of this product is not recommended when breastfeeding.
4.7. Effects on ability to drive and use machines
Not applicable.
Minor gastrointestinal effects such as nausea, vomiting, gastralgia, pyrosis, dyspepsia have been reported infrequently.
- Have been described:
o cases of mucocutaneous reactions such as erythema, rash, pruritus, urticaria;
o very rarely, anaphylactoid reactions with shock and angioedema, which have had a favorable outcome in reported cases.
In these cases, treatment must be discontinued.
- Headache and dizziness have also been reported very rarely.
In case of overdose, treatment should be symptomatic.
5.1. Pharmacodynamic properties
Pharmacotherapeutic class: MUCOLYTICS, ATC Code: R05CB06.
Ambroxol has mucokinetic and expectorant properties.
It stimulates, by its action on the secretory cells, the bronchial secretion and promotes the production of a more mobilizable mucus. It increases ciliary activity.
5.2. Pharmacokinetic properties
Ambroxol is well absorbed orally. Peak plasma levels are reached in approximately two hours.
Bioavailability is approximately 70%.
High volumes of distribution indicate significant extravascular distribution.
The elimination half-life averages 7.5 hours. Elimination is predominantly urinary, with two major metabolites excreted as glucuronide conjugate.
Not applicable.
Glycerol, citric acid monohydrate, methyl parahydroxybenzoate (E218), propyl parahydroxybenzoate (E216), banana flavour, liquid sorbitol (non-crystallisable), purified water.
Not applicable.
2 years.
6.4. Special precautions for storage
No special precautions for storage.
6.5. Nature and contents of outer packaging
100 ml or 150 ml in a bottle (type III brown glass) closed with a stopper (Polyethylene) fitted with a seal (Polyethylene) with a measuring cup (Polypropylene) graduated at 2.5 ml, 5 ml, 7.5 ml, 10 ml and 15 ml or a measuring spoon (Polystyrene) graduated at 2.5 ml and 5 ml.
6.6. Special precautions for disposal and handling
No special requirements.
7. MARKETING AUTHORIZATION HOLDER
BIOGARAN
15 BOULEVARD CHARLES DE GAULLE
92700 COLOMBES
8. MARKETING AUTHORISATION NUMBER(S)
- 361 787-6: 150 ml bottle (glass) + measuring cup (Polypropylene).
- 361 920-8: 150 ml bottle (glass) + measuring spoon (Polystyrene).
- 381 268-4 or 34009 381 268 4 6: 100 ml bottle (glass) + measuring spoon (Polystyrene).
- 381 415-7 or 34009 381 415 7 3: 100 ml bottle (glass) + dosing cup (polypropylene).
9. DATE OF FIRST AUTHORISATION/RENEWAL OF AUTHORISATION
[To be completed by the holder]
10. DATE OF UPDATE OF THE TEXT
[To be completed by the holder]
Not applicable.
12. INSTRUCTIONS FOR THE PREPARATION OF RADIOPHARMACEUTICALS
Cuts, bumps, bruises, contusions. Calms and soothes. Enriched with arnica extract and essential oil. From 3 years.
Indications : Short-term treatment of acute diarrhoea in adults (from 15 years old). This treatment is a complement to dietary measures: read carefully the chapter "Taking special precautions".
Indications . Reserved for infants up to 30 months of age. Traditionally used in benign acute bronchial affections.
Indications: This medicine is indicated for sore throats that are not very intense and without fever.
Glycerin suppositories for adults from Cooper Laboratories are intended for the symptomatic treatment of low-level constipation, particularly due to rectal dyschezia, and in preparation for endoscopic examinations of the rectum.
Available in boxes of 25, 50 or 100 glycerine suppositories.
DulcoSoft 2 in 1 is a medical device designed to relieve the symptoms of occasional constipation and reduce the sensation of bloating. It comes in the form of a powder to be mixed with water to make an oral solution. Dulcosot 2 in 1 is tasteless.
Symptomatic treatment of mild to moderate pain and/or febrile states.
Indications: This medicine is recommended for the treatment of certain diseases of the heart and vessels.
Chlorhexidine 0.12% Mylan Mouthwash Solution is a medication indicated for the adjunctive treatment of infections of the mouth and care after surgery
Chlorhexidine 0.12% is an antiseptic that treats oral infections. It is also used for post-operative care in odonto-stomatology.
Syntholkiné Heating Patches have been designed to relieve muscle pain through heat: thermotherapy, an effective and natural solution used regularly by physiotherapists.